The Maillard reaction and coffee beans
For many people a hot brewed cup of coffee is a morning essential and also a reliable companion through the working day. The stimulating taste and smell aromas are created in the roasting process of the coffee beans during the so-called Maillard reaction(1)(2) In this important chemical reaction, reducing sugars react with amino acids to form Schiff’s bases. After several rearrangement reactions, these bases form highly reactive carbonyl compounds, from which many other reaction products, including heterocyclic compounds, can be formed. The products of the Maillard reaction are therefore responsible for the taste, smell and texture of many foods such as fried products, bread, cookies, grilled meat or even coffee.
But there are also undesired by-products of the Maillard reaction. One of these is acrylamide, which is formed when asparagine reacts with glucose. High doses of acrylamide have been shown to be carcinogenic and mutagenic in animal experiment(3-6). 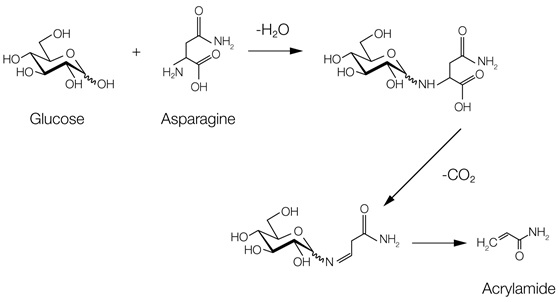
Clear evidence of a carcinogenic effect also in humans is still missing(7). Nevertheless, it is advised to avoid the consumption of acrylamide if possible.
MACHEREY-NAGEL products help to analyze the acrylamide content in coffee.
The application note describes the sample clean-up with either non-dispersive or dispersive dSPE (“QuEChERS”) and subsequent HPLC-MS/MS analysis.
Access to application note: Determination of acrylamide in coffee
References:
(1) L. C. Maillard: Formation of Melanoidins in a Methodical Way.. In: Compt. Rend.. 154, 1912, S. 66
(2) J. E. Hodge: Dehydrated Foods, Chemistry of Browning Reactions in Model Systems. In: Journal of Agricultural and Food Chemistry. 1, Nr. 15, 1953, S. 928–43. doi:10.1021/jf60015a004
(3) Dearfield KL, Abernathy CO, Ottley MS, Brantner JH, Hayes PF. Acrylamide: Its metabolism, developmental and reproductive effects, genotoxicity, and carcinogenicity. Mutation Research 1988; 195(1):45–77.
(4) Dearfield KL, Douglas GR, Ehling UH, et al. Acrylamide: A review of its genotoxicity and an assessment of heritable genetic risk. Mutation Research 1995; 330(1–2):71–99.
(5) Friedman M. Chemistry, biochemistry, and safety of acrylamide. A review. Journal of Agricultural and Food Chemistry 2003; 51(16):4504–4526.
(6) National Toxicology Program. Toxicology and carcinogenesis studies of acrylamide (CASRN 79-06-1) in F344/N rats and B6C3F1 mice (feed and drinking water studies). National Toxicology Program technical report series 2012; (575):1-234.
(7) Pelucchi C, La Vecchia C, Bosetti C, Boyle P, Boffetta P. Exposure to acrylamide and human cancer - a review and meta-analysis of epidemiologic studies. Annals of Oncology 2011; 22(7):1487-1499.






