In the research of effective therapeutics against SARS-CoV-2, the focus is repeatedly shifting to active ingredients that have achieved good success in treating already known (viral) infectious diseases such as HIV, Ebola, SARS, and MERS in affected patients. This also includes the broad-spectrum antiparasitic Ivermectin, an FDA-approved drug against worm infestation, which has also been shown to have anti-viral activity in suppressing HIV-1 replication.(1)
Ivermectin was added to SARS-CoV-2 infected cells in an in vitro experiment. The subsequent PCR replication of the SARS-CoV-2 RNA, showed that the viral activity had decreased by > 90%.(2)
However, to date there is no evidence of therapeutic efficacy through in vivo experiments or proof of clinical benefit against SARS-CoV-2.
These pre-published results prompted the U.S. Food and Drug Administration to issue a warning that Ivermectin is only an FDA-approved drug for the treatment of certain parasitic diseases in animals. Under no circumstances should Ivermectin tablets be consumed by humans for self-medication or prevention of COVID-19.(3)
Chemically speaking, Ivermectin is a complex macrocycle with a lactone skeleton (macrolide) consisting of a mixture of two compounds, H2B1a (ca. 90%) and H2B1b (ca. 10%), which differ structurally only in one methyl group.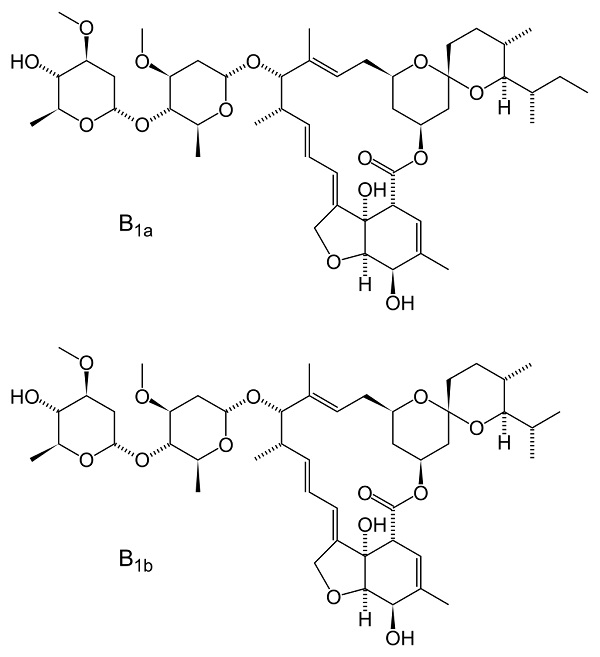
The HPLC identification was performed according to the specifications of the European Pharmacopoeia.
Access to HPLC application: Ivermectin according to EP conditions
Further examples of drug analysis related to COVID-19
References:
(1) Wagstaff, K.M., et al.,2012. Biochem. J. 443 83), 851-856
(2) L. Caly, J.D.Druce, M. G. Catton, D.A. Jans, K:M. Wagstaff, Antiviral Research 178 (2020) 104787
(3) https://www.fda.gov/animal-veterinary/product-safety-information/fda-letter-stakeholders-do-not-use-ivermectin-intended-animals-treatment-covid-19-humans