Qualitative pH test paper Congo paper MN 816 N, pH: 5.0–3.0

*taxes and shipping not included
Delivery time approx. 5 working days
Red paper with normal sensitivity, which changes color to blue in the pH range 3.0–5.0, e.g. for neutralization analysis, detection of lactic acid in culture media, which contain lactic acid-producing strains of B. coli.
| Platform | Products for pH determination |
| CE certified | No |
| Parameter | pH |
| Detection limit | pH - 5.0–3.0 |
| Color change | pH - red → blue |
| Width | 7 mm |
| Shelf life (from production) | 2 Year(s) |
| Storage temperature | 4–30 °C / 39–86 °F |
| Scope of delivery | 1 reel of 5 m length and 7 mm width |
| Gross weight (incl. packaging) | 25.16 g / 0.06 lbs |
| Packaging dimensions | 63 x 18 x 63 mm / 2.48 x 0.71 x 2.48 Inch |
| Hazardous material | Yes |
pH
The pH-value indicates whether a water or solution is acidic, alkaline or neutral.
pH-testing using test strips and test papers
A variety of test strips and test papers is available to easily determine the pH of a sample.
Overview pH-testing products

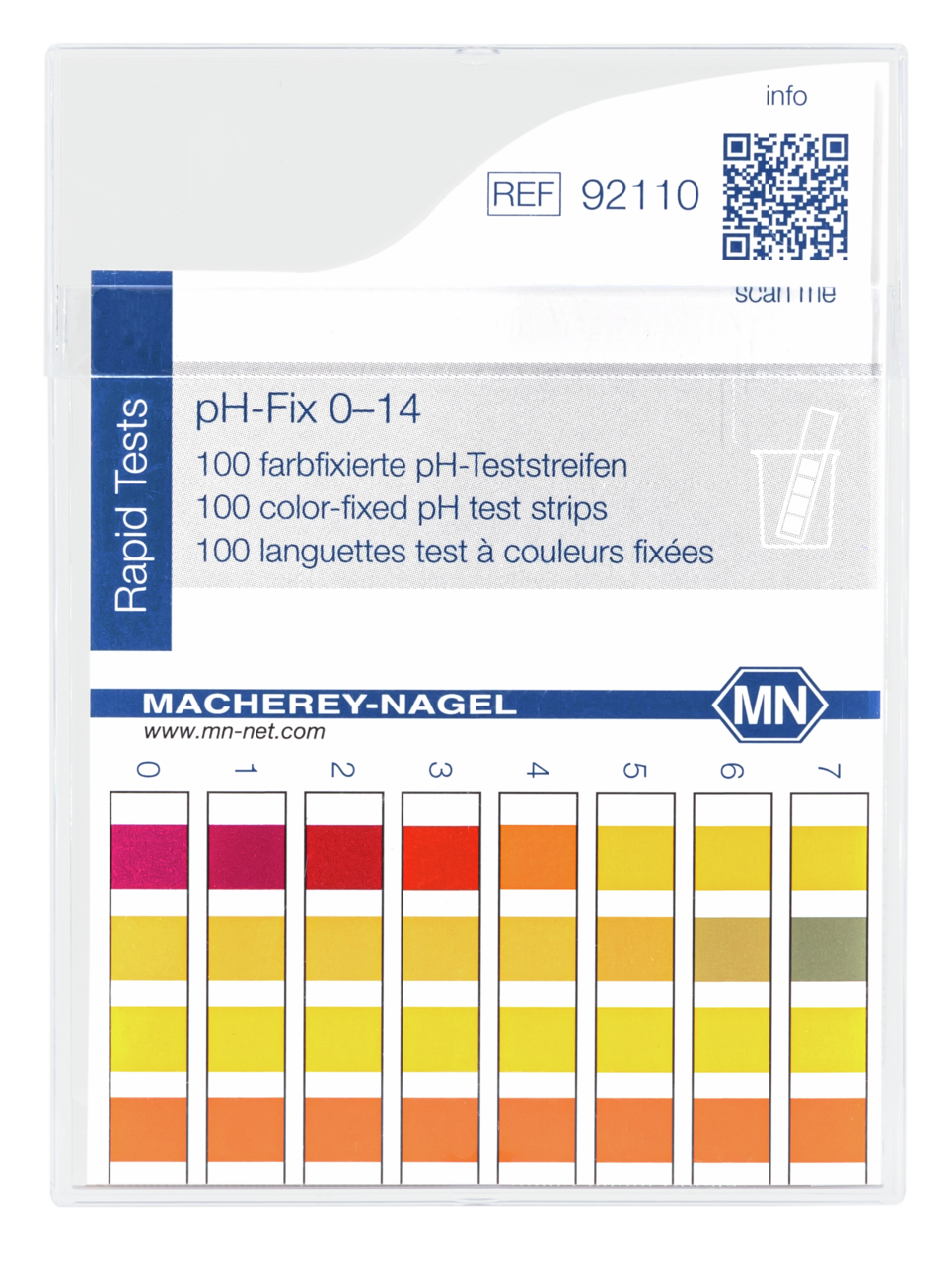
pH-Fix – Unmatched pH test strips
For many years, untrained users as well as analytical professionals appreciate the experience of easy pH-testing with pH Fix. In contrast to common indicator papers, the indicator dyes in pH Fix test strips are chemically bound to the test pads. This technology prevents bleeding of the dyes and therefore a contamination of the sample, even in highly alkaline solutions. The fixation enables the strips to remain in solution over extended periods allowing a safe pH determination even in weakly buffered solutions.
PEHANON – pH determination in colored solutions
PEHANON test strips unify pH indicator and reference color chart on one strip. Any sample color has the same effect on both, the reference colors and the reactive pad, allowing unadulterated pH reading even in colored solutions.
pH indicator papers - standard for many applications
pH indicator papers have been available for decades and are the standard for many applications. For each pH value, these papers show a single color that can be matched with the color scale at intervals of 0.2–1 pH units. The indicator papers come in plastic reels that ensure long-term stability and protection against many external influences. They will be always ready-to-use when needed.


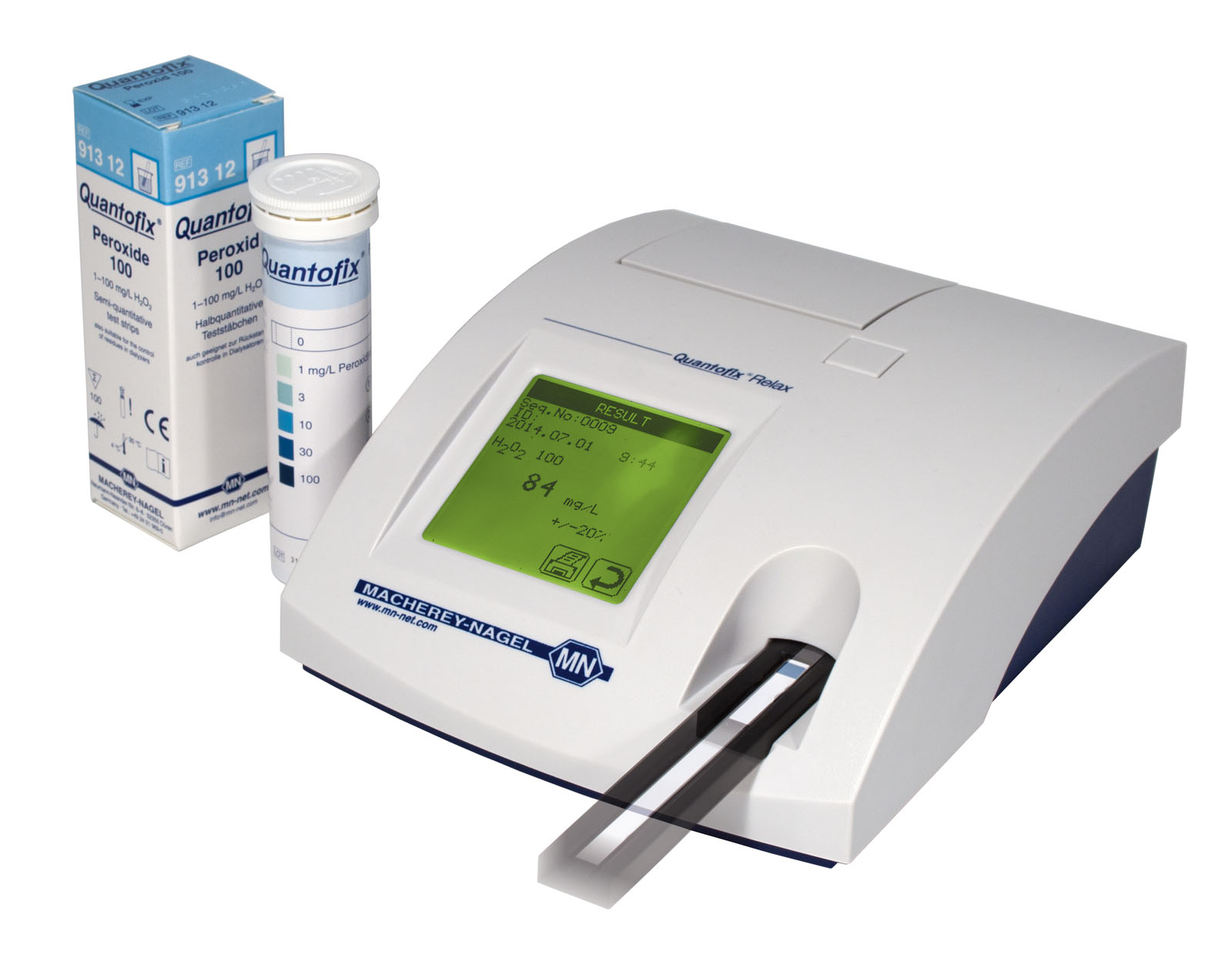
Documented results with QUANTOFIX Relax
The strip reader QUANTOFIX Relax provides objective pH-readings with a selection of pH-Fix test strips. Measurement data including time date and sample ID are printed, stored and can be transmitted to an information system. This allows the rapid and reliable documentation of test results, which proved to be especially useful for QC departments.
Best pH-Fix product compared to competitor!
According to a comparative study, our MACHEREY-NAGEL pH-Fix 0-14 test strips
performed as the best! In various tests our strips delivered the most reliable
pH values and the best marks in usability compared to the competitor product.
Perfect color matching and clear design
The brilliant color scale on the test strip box allows an exact comparison with the
color development on the test fields. Above all, the clear design enhances the intuitive and fast determination of the pH value.
Longest test strips in comparison
The long hydrophobic plastic strip protects the user from hazardous or aggressive
samples and allows pH tests in narrow and deep vessels. Especially in handling, the long test strip proves to be a great advantage compared to other products!
Patented technology prevents contamination
The indicator is covalently bound to the test field and does not bleed into the sample. Therefore the test strips can remain in the sample without contaminating it.
Check out our comparative study and convince yourself of the best pH test strip on the market!
See for yourself! Try the MACHEREY-NAGEL quality.
Easy sampling
Sampling is often the biggest source of error in the analytical process. In general, the best practice is to draw an aliquot of the sample and measure the pH in this aliquot. However, when analyzing inhomogeneous samples, drawing an aliquot may alter the composition of the sample. In this case, it is often better to dip the strip directly into the sample. Make sure, to not remove particles from the sample with the test strip. Use pH-Fix test strips only, because indicator papers may release small portions of indicator into the sample.
General information on pH
pH values play an important role in chemistry, engineering, and biological processes. Many chemical reactions, particularly chemical equilibrium reactions, are strongly influenced by the pH. In many such processes, exact determination and appropriate regulation and compliance with the specified pH is required. Thus, e.g. the EC Directive on the Quality of Water Intended for Human Consumption specifies a pH range between 6.5 and 9.5. To achieve such a pH, the water can be treated with commercially available chemicals.
Normal waters usually have a pH of about 6.5 to 7.5. Domestic sewage is neutral to slightly alkaline, and commercial wastewaters tend to be more acidic, e.g. the pickling waste of the iron working industry.
The definition of the pH is based on the so-called autoprotolysis of pure water. At room temperature, pure water is to a small extent split (dissociated) into equivalent amounts of hydrated hydrogen ions (hydronium ions) H3O+ and hydroxide ions OH–:
| 2 H2O → H3O+ + OH- |
The product of the concentrations of H3O+ and OH– is referred to as “ionic product of water” and can be assumed to be an approximately constant value:
| c(H3O+) · c(OH-) = 10-7 mol/L · 10-7 mol/L = 10-14 mol2/L2 (at 25°C) |
Using this ion product, for any given concentration of H3O+ ions (from now on referred to as H+) or OH– ions the respectively associated unknown concentration can be calculated. According to Brønsted, acids act as H+ donors and bases as H+ acceptors. For example, addition of acids to the water increases the concentration of H+ ions, while that of OH– ions decreases accordingly. The pH was introduced to simplify the notation of low concentrations and for better comparison.
H2O = Water
H3O+ = Hydronium-Ion
OH- = Hydroxide-Ion
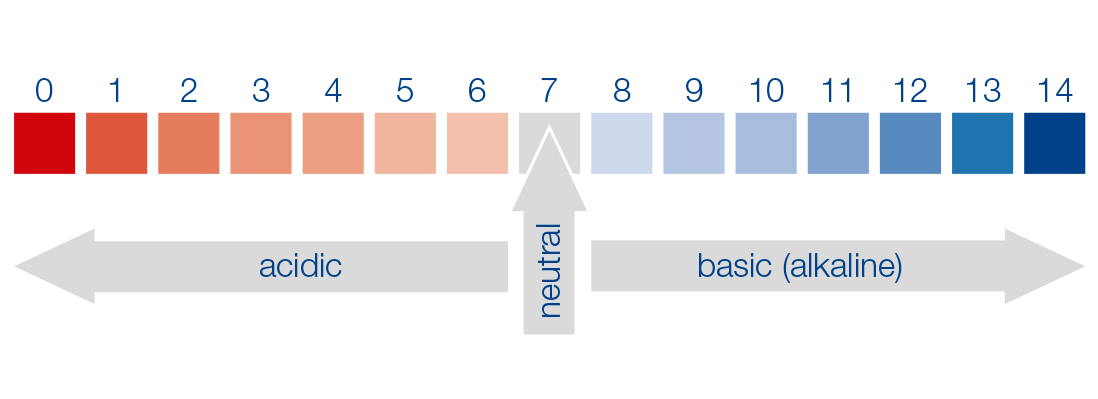
The pH is thus a measure of the acid or base strength of an aqueous solution. By definition, an aqueous solution is acidic in the pH range < 7 (high H+ concentration, low OH– concentration) and basic (alkaline) in the pH range > 7 (low H+ concentration, high OH– concentration). A pH of 7 (the so-called neutral point) thus means that the concentration of H+ ions corresponds to that from the dissociation of pure water (in equilibrium with the OH– ions).
Mathematically, the pH (lat.: potentia Hydrogenii) is defined as the negative decadic logarithm of the numerical value of the H+ activity given in mol/L, and is derived from the ion product (autoprotolysis of water) of water (as a first approximation, the H+ ion concentration [H+] can be used in the calculation), so that a numeric scale from 0 to 14 is obtained. The pH is a dimensionless unit.
| pH = - log c(H3O+) |
Strong acids and bases, such as hydrochloric acid, HCl, or sodium hydroxide NaOH (caustic soda), are (almost) completely dissociated in solution, i.e. split into their ions. Thus they have a strong influence on the change in concentration of H+ and OH– ions, which results in a low (0–3) or high (11–14) pH. Weak acids (e.g. acetic acid, CH3COOH) and bases (e.g. ammonia, NH3) are not completely split into their ions in water and therefore affect the pH to a lesser degree.
Salts of weak acids and strong bases (e.g. sodium carbonate, Na2CO3) or of weak bases and strong acids (e.g. calcium chloride, NaHSO4) likewise result in pH shifts upwards or downwards, respectively, by hydrolysis (reaction with water / splitting into individual ions). If, however, a salt, such as sodium chloride, NaCl, is dissolved in water, the pH will not change (or only barely), since no new hydrogen ions H+ are produced or consumed (sodium chloride splits into the ions Na+ and Cl–).
The correct pH is an important factor for many detection reactions. The reactions will be affected, if the pH value is not properly adjusted. In most cases, the detection reaction is less selective or does not proceed at all.
Another important role of pH is in biological wastewater treatment. If the pH of 6.0 to 8.0 is not maintained correctly, the bacteria will die, and the purification cannot be performed.
An excessive pH (above 9) over a longer period in the activation tank leads to increasing ammonia content of the water. Inhibition of nitrification or carbon degradation may result. Too high or too low pH levels can also lead to a decomposition of flakes. Proper separation of filterable precipitates is no longer possible.
The pH is changed, especially in sewage treatment plants, by entry of certain detergents, toilet cleaners (WC stones) or industrial effluents. The pH should be checked at various stages of degradation.
The pH is a dimensionless value.
The pH is a dimensionless value.
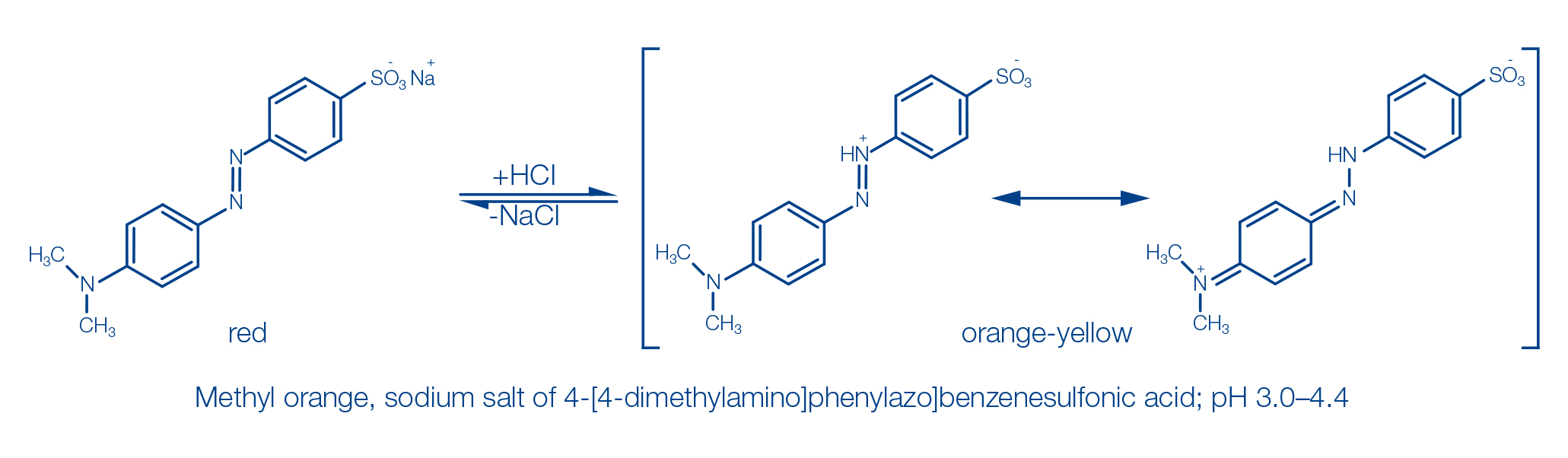
Reaction basis
pH indicator dyes are chemical compounds whose color changes depend on the pH. Most of them are complex organic molecules. Indicator dyes are acids or bases themselves and thus able to release or absorb protons.
The color change of the indicators is performed under the influence of the changing concentration of H+ ions by alteration of the chemical structure, where especially occurrence of quinoid structures or conjugated double bonds is of importance. An example of this is the indicator dye methyl orange, which shows a change in color from red to orange-yellow in the pH range 3.0–4.4.
pH indicator dyes are chemical compounds whose color changes dependent on the pH.
Indicator dyes belong to various organic dye classes (e.g. azo dyes, phthaleins, sulfophthaleins, benzeines, triphenylmethyl dyes, nitro indicators, etc.). The transition range of the individual indicators extends over 1.2 to 2.5 pH units. Beyond these limits, hue and color depth do not change further.
A special mixture of different indicator dyes shows a characteristic color at any pH. Mixtures of indicator dyes are often referred to as universal indicators, since a combination of appropriate indicators covers large ranges of the pH scale, or even the entire range of 0–14.
Photometric pH determination (VISOCOLOR and NANOCOLOR) takes place in water with phenol red, a triphenylmethane dye, as an indicator. Easy pH measurement is possible with pH indicator strips (pH-Fix).
In strongly acidic media (pH < 1), phenol red shows a red color, at a pH of 1–7.3 a yellow color, while in slightly basic medium (above pH 7.3) the indicator turns purple, and in strongly basic medium (pH > 14) phenol red is colorless. The individual colors are due to the chemical structure.
Sample preservation
The sample should be measured quickly within one day. Long-term preservation is not possible.
Tips & tricks
Common sources of error
- Incorrect measurement results due to failure to observe the influence of temperature:
The ion product of water is temperature-dependent. The degree of dissociation, i.e. the percentage of dissociated molecules, increases strongly with temperature. All test papers and indicator strips are calibrated to standard solutions of 20 °C.
- pH measurements in colored solutions:
pH measurements in colored solutions always require a special treatment. Theoretically, according to the liquid to be examined, the reference solution should be adjusted to the same color. The same applies to a turbid solution. MACHEREY-NAGEL offers a special form of pH measurement in colored solutions in the form of the PEHANON indicator papers, where the color comparison scale is subject to the same shifts and influences during the measurement as the comparison field is. Stains and turbidities are thus compensated. Color compensation is also done in the colorimetric VISOCOLOR tests.
- Sources of error that may arise from the contents of the solution to be examined:
- Acid-base error
In terms of their chemical nature, acid-base indicators are themselves acids or bases of more or less pronounced character. Consequently, their very addition to non-buffered or only very weakly buffered solutions (e.g. distilled water, neutral salt solutions, solutions of poorly hydrolyzed salts, very weak acids or bases, very dilute solutions of strong acids and salts) will result in a certain change in pH. This error is called acid or base error, respectively, depending on whether the indicator is an acid or a base. These errors are by no means negligible; in the worst cases, these errors can exceed more than one pH unit.
For this reason, caution is advisable when measuring pH values in non-buffered or weakly buffered solutions. - Salt error
Ions other than hydrogen ions H+ likewise cause, albeit small, effects on the color development of the indicators. This can lead to color differences in pH measurements in different salt solutions. This effect is known as “salt error”. At a salt concentration < 0.2 mol/L, a relevant correction can be neglected. - Alcohol error
When using solvents other than water, the position of the acid-base balance and thus also the indicator constant is changed. This means that in direct color comparison of an indicator in an aqueous buffer solution with a solution containing a small amount of alcohol, identical color does not necessarily imply identical pH values of the two liquids. At room temperature, the alcohol error may be up to 0.5 pH units (indicator-dependent). - Protein error
Proteins are amphoteric in character, having both acidic and alkaline properties. Thus, proteins bind indicators with acidic as well as with basic character, whereby the resulting color is affected. Thus, pH determination in protein-containing solutions is often very difficult or even impossible. The error is dependent on the type and quantity of the protein, as well as on the nature of the indicator. - Alkaloid error
Alkaloids are also capable of forming conglomerates with certain indicators. If alkaloids are present, it is recommended to perform blank value determinations in order to control the influence of the alkaloids on the measurement.
Amphoteric compounds may react in one manner or the other, e.g. as acids and bases.
Background information
- Generally, pure water and neutral salt solutions are very sensitive to atmospheric carbon dioxide. Air contains about 0.03 % v/v of carbon dioxide. In equilibrium with air, distilled water absorbs carbon dioxide. Therefore, under normal conditions distilled water does not exhibit a neutral pH of 7.
- Buffer solutions are required to achieve constant pH adjustment. Buffers are solutions of a weak acid and one of its corresponding salts (e.g. acetic acid / acetate buffer) or a weak base and one of its corresponding salts (phosphate / phosphoric acid buffer). The pH of such solutions will change little or not at all in case of dilutions, or even upon the addition of stronger acids or bases, in the pH range defined for them. It should be noted here that all buffer solutions have a certain maximum buffer capacity. Once this is “used up”, the amount added exceeds the availability of the consumed amount of buffer. For pH measurement using indicator papers, above all adequate buffering is necessary.
Sea water suitability
All VISOCOLOR and NANOCOLOR pH tests are suitable for seawater analysis.
 Qualitative pH test paper Congo paper MN 816 N, pH: 5.0–3.0, refill pack
Qualitative pH test paper Congo paper MN 816 N, pH: 5.0–3.0, refill pack 





